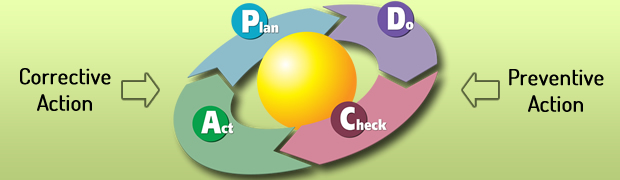
The regulatory landscape continues to change in today’s business world. One of the systematic quality approaches defined by regulators and the industry for mitigating risk is corrective and preventive action, better known as CAPA. Businesses and organizations are often challenged by the CAPA process, which is one of the most important and essential elements of a quality management system. An effectively implemented and integrated CAPA program, can benefit in all areas of an organization and inadequate execution of a CAPA strategy continues to be one of the most common nonconformities cited during audits.
Common CAPA pitfalls:
- Implementing CAPA rather analysing the actual root cause
- Driving CAPA process than on deliverables
- Setting unrealistic timeframes
- Using same root cause analysis tool
- Delays in actions and decisions
A well-organized CAPA program within the quality management system (QMS) help businesses to identify, track, correct and prevent recurrence of non-conformities. Proper CAPA strategy helps businesses to resolve bugs, errors, anomalies and issues. So It is essential component in any QMS to maintain quality as well as the existing quality standards. Paper-based systems may be cost-effective but they are inefficient and require extensive man-hours for issue resolution. Automated QMS system will save more on time and helps to conclude discrepancies with actual resolution of the CAPA
Benefits of having QMS for CAPA:
- Decreased cost of quality (scrap, nonconforming products, complaints, field actions, process deviations)
- Minimal resource utilization
- Enhanced communication
- increased process efficiencies
- improved traceability
To get further more details about CAPA and Automated QMS solutions Contact us @www.auraqualitymanagement.com